 This fall, TLC presented data at the American Society of Anesthesiologists annual meeting from a Phase I/II clinical trial which showed TLC590 to yield more immediate and long-lasting pain reduction than ropivacaine.
This fall, TLC presented data at the American Society of Anesthesiologists annual meeting from a Phase I/II clinical trial which showed TLC590 to yield more immediate and long-lasting pain reduction than ropivacaine.
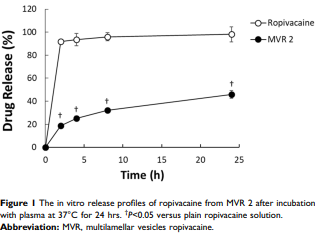
The multilamellar vesicles ropivacaine (MVR) demonstrates a sustained-release profile in an in vitro serum environment model after 24 hrs of incubation which translates in the in vivo rat pharmacokinetic profile of ropivacaine as a prolonged half-life that is 10-fold longer in duration than plain ropivacaine solution. The anesthetic effect of single-dose MVR is apparent by providing a prolonged analgesia effect compared to plain ropivacaine solution in an in vivo guinea pig pin-prick wheal model after a single intracutaneous injection.
From a safety evaluation, MVR is well tolerated after a subcutaneously injection at a dose level of 20 mg/kg in rats, with no observable changes in clinical observation, body weight, organ weight, hematology and serum chemistry analysis In addition, in vivo findings in which TLC590 showed no dose-related toxicity and other preclinical data were recently published in the International Journal of Nanomedicine.
OAS members have long expressed interest in non-opioid solutions for pain management following surgery, so we’ll stay tuned for more advances – including advances in nanoscience and bioengineering.
Read the journal article online.